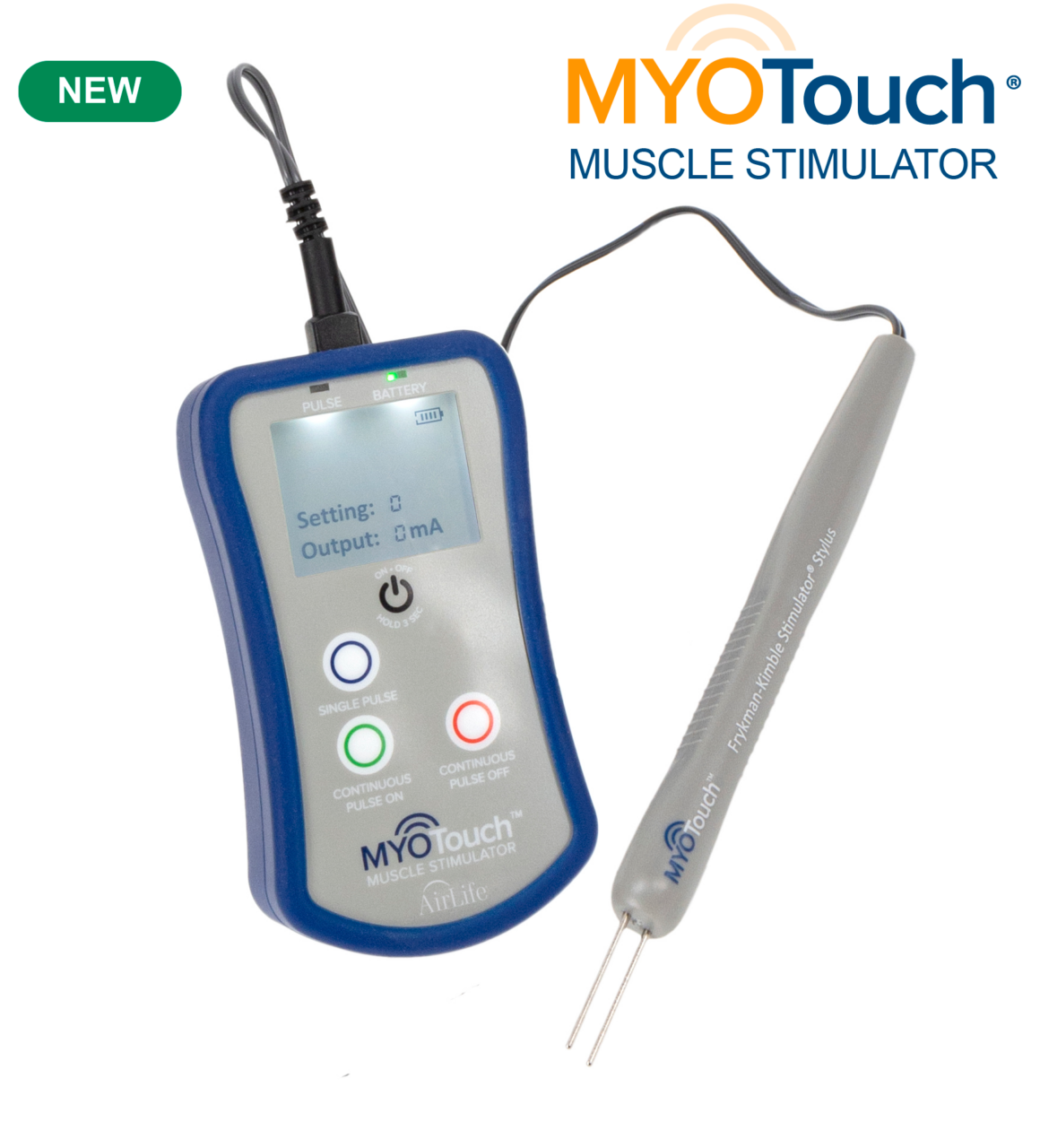
Introducing MYOTouch® Muscle Stimulator
AirLife and GPSTEP are Partnering to Bring Effective Surgical Care to Children Globally
- Innovative device designed specifically for anorectal malformation repair
- FDA cleared for use on pediatric patients, birth through adolescence
- Purchases help bring MYOTouch to underserved regions of the world
*You will be redirected to Mainline Medical to order MYOTouch.
AirLife is pleased to have partnered with Dr. Philip K. Frykman, MD, PhD, MBA, an internationally recognized leader in the field of pediatric surgery on the development of MYOTouch.
We are proud to continue this partnership by helping to bring this critical device to surgeons working with affected children in underserved regions around the world.
MYOTouch® provides precise muscle stimulation to aid surgeons in accurately identifying muscles essential for successful anorectal reconstructions.
It can be used for complex reconstructive surgeries such as Posterior Sagittal Anorectoplasty (PSARP) and laparoscopic repair of anorectal malformations (ARM).
The Need for MYOTouch is Real
MYOTouch Can Help in Critical ARM Repairs
According to the National Institutes of Health, US Department of Health and Human Services, about one in every 5,000 neonates is born with an anorectal malformation (ARM), which are defects in the formation of the anus and rectum. These malformations can result in a variety of functional and anatomical challenges, such as difficulties with bowel movements and the absence of an anus.
Posterior Sagittal Anorectoplasty (PSARP) surgery is a crucial procedure for children born with anorectal malformations. ARMs are one of the most challenging congenital malformations in pediatric surgery and prompt treatment is crucial for babies born with this malformation.
The MYOTouch is the only FDA cleared muscle stimulator for PSARP and laparoscopic repair of ARMs, from birth through adolescence.
PSARP surgery helps restore anatomical functionality through reconstructing the anus and rectum to allow for normal bowel movements. It addresses issues like the improper placement of the anus, or absence of an anal opening. Additionally, the procedure helps correct anatomical defects by restoring the proper anatomical structure of the anus and rectum. PSARP helps improve quality of life for affected patients by restoring health and function of their anatomy.
MYOTouch is uniquely designed to aid surgeons in identifying striated muscles and delivering precise muscle stimulation, essential for successful anorectal reconstructions. The sterile, single use Frykman-Kimble Stimulator Stylus is ergonomically designed for ease of use and control while the tips are designed for use on delicate tissue. MYOTouch can deliver single or continuous pulse stimulation at the surgeon’s discretion.
Overall, PSARP surgery is a vital treatment for children with anorectal malformations, providing both immediate and long-term benefits in terms of health, function, and quality of life. AirLife is pleased to bring MYOTouch to pediatric surgeons for use in PSARP procedures.
Easy to Order
MYOTouch is available to order direct from Mainline Medical, our exclusive, authorized distributor for MYOTouch.
Click or Call to Order MYOTouch
*You will be redirected to Mainline Medical to order MYOTouch.
Have Additional Questions?
For any questions about MYOTouch or to request further information, please reach out to us at myotouch@myairlife.com.
Evolution of a Revolutionary Device
Since 2011, Dr. Frykman and his colleagues have been dedicated to addressing the surgical needs of children affected by anorectal malformations. He has seen first-hand the need for a muscle stimulator to effectively support anorectal procedures.
Pediatric surgeon Dr. Philip Frykman and pediatric anesthesiologist Dr. Keith Kimble travel to China to perform anorectal malformation (ARM) repairs on affected babies utilizing improvised methods.
Recognizing the current limitations and cost barriers associated with existing solutions, the doctors conceptualize a dedicated, affordable medical device specifically designed to streamline and enhance ARM procedures.
The European Journal of Pediatric Surgery publishes an article hypothesizing that low-cost nerve stimulator is equally effective as a high-cost muscle stimulator for ARM surgery (authored by Drs. Frykman, Kimble et al). The article prompts a flood of inquiries from physicians in Cuba, Indonesia, Nicaragua, China and more. The demand for an ARM repair device is evident.
The team sets their sights on developing a low-cost muscle stimulator device that is sterilizable and durable with worldwide distribution potential. The Global Pediatric Surgical Technology and Education Project (GPSTEP) is founded by Drs. Frykman and Kimble plus colleagues to bring their vision of a muscle stimulator for ARM surgeries to life and make it available globally.
Prototypes are made with the goal of developing a functional, durable and reusable probe. This is supported in part through a grant from Mending Kids. The team conducted procedures and trainings with the device in South Africa.
The GPSTEP team began their search to find a partner to help them develop a muscle stimulator for use and sale in the US market.
Drs. Frykman and Kimble approach approached SunMed (now AirLife), hoping to enlist their engineering and quality management expertise for the next ARM muscle stimulator.
AirLife recognizes the critical nature of this unique need in pediatric medicine. Together, AirLife Engineers work closely with Dr. Frykman to enhance the functionality and use of the original stimulator device and redesign the probe to be a single-use sterilized stylus. AirLife facilitates all functional and safety testing, builds prototypes and packaging.
The AirLife Quality Management team expertly navigates the FDA medical device application process for the ARM device and secures 510K approval for the MYOTouch Muscle Stimulator.
- 2011
- 2012
- 2013
- 2013
- 2014
- 2017
- 2020
- 2021
- 2024
- 2025
Global Pediatric Surgical Technology & Education Project (GPSTEP)
AirLife is proud to partner with the Global Pediatric Surgical Technology and Education Project (GPSTEP) in addressing the surgical needs of children worldwide. AirLife will be donating a portion of proceeds from MYOTouch purchases plus devices to GPSTEP to support distribution of MYOTouch Muscle Stimulators to pediatric surgeons in developing countries and underserved populations. GPSTEP is a 501(c)(3) organization based in Irvine, California and founded in 2013 by Drs. Philip Frykman (pictured left) and Keith Kimble (pictured right).




